Aroa Biosurgery expects its Myriad portfolio to continue driving strong growth in the soft tissue reconstruction market
Initially focus on trauma segment of market, which based on its estimates has a TAM in the US of ~US$300 million
Clinical studies show Myriad enables patients to build tissue rapidly, which is important in trauma cases
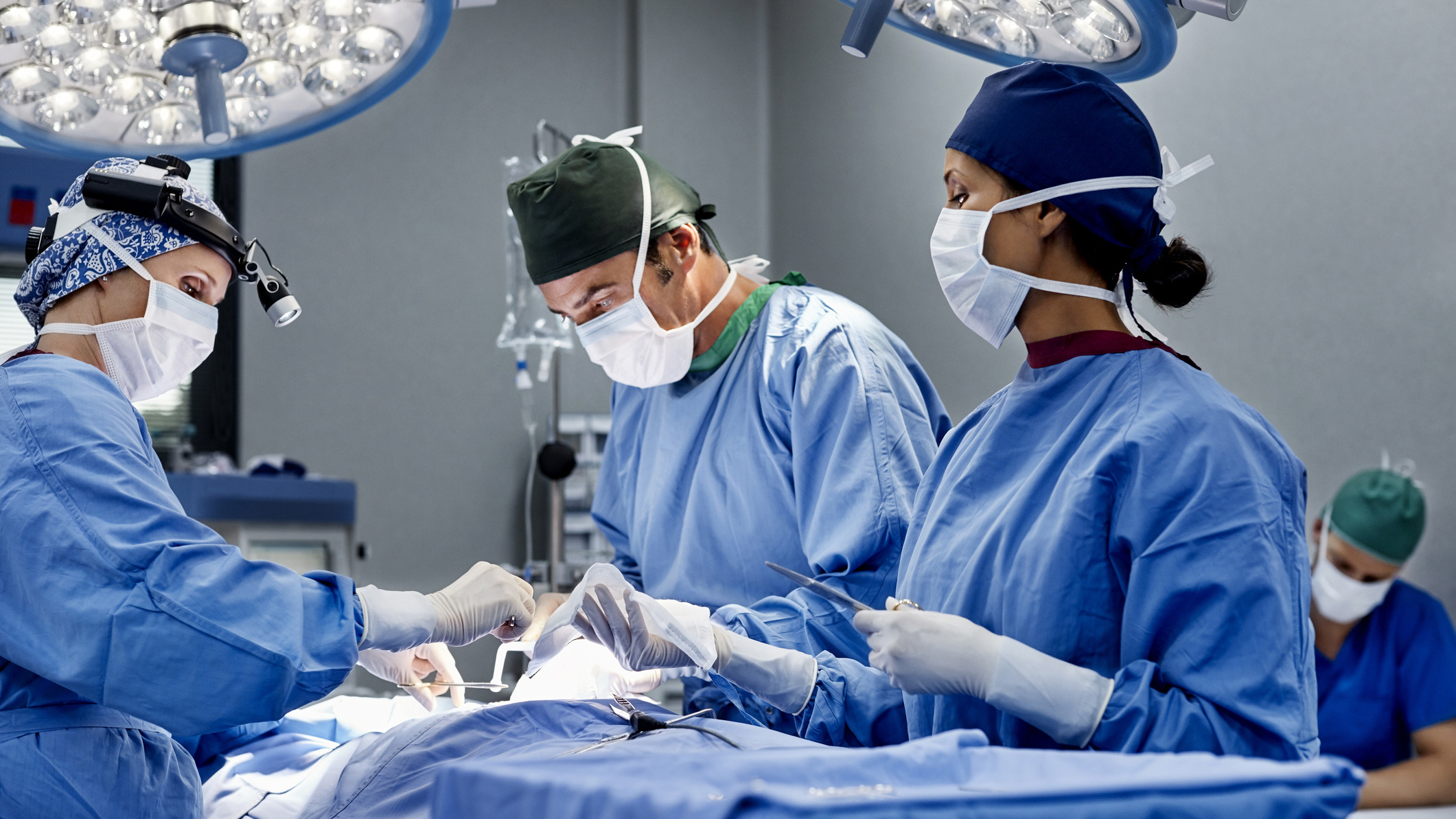
Aroa Biosurgery (ASX:ARX) is putting increased focus on opportunities in trauma following launch of its Myriad family of products with its direct sales force three years ago leading to increased growth in trauma use and encouraging preliminary data from clinical studies.
The Myriad family of products, based on its proprietary tech using ovine forestomach tissue, supports soft tissue reconstruction in a wide range of surgical procedures.
Early results in a study at the Department of Trauma and Acute Care Surgery, WellStar Kennestone Hospital in the US state of Georgia has demonstrated that Myriad is effective in emergency open abdomen surgery, where the abdominal cavity is exposed and closing these wounds requires new tissue to develop. This can be very difficult to achieve.
ARX says while there are only three cases in this study, there was 100% graft integration, meaning the product transformed into new tissue successfully within in a mean time of 19.3 days, with a mean overall healing time of nine weeks.
The results reinforce a growing body of clinical evidence supporting the efficacy of Myriad in complex trauma procedures. Additionally, Myriad was successfully combined with negative pressure wound therapy, a frequently used treatment modality in trauma procedures.
Another peer-reviewed retrospective pilot case series released in October 2023 outlined the clinical effectiveness of Myriad Matrix and Myriad Morcells in complex traumatic wound reconstruction procedures.
The retrospective case series assessed 13 complex traumatic wounds across 10 patients, including injuries from motor vehicle accidents, abdominal dehiscence following hernia repair, Fournier’s Gangrene, compartment syndrome and pressure injuries, at a single US Level 1 trauma centre, between January 2021 and February 2023.
The study found the average time to soft tissue coverage and fill was 23.4±9.2 days, with a median product application of 1.0.
No complications were reported from the study cohort.
CEO Dr Brian Ward told Stockhead the company also has a Myriad registry running in the US with 300 patients enrolled.
“We expect the registry to continue to generate evidence to support the use of Myriad in trauma and in combination with Negative Pressure Wound Therapy over the next 12 months,” he says.
“Our goal is to demonstrate that Myriad improves healing times and reduces the costs of treating patients for hospitals.
“These upcoming studies and a planned pilot study this year are laying the foundations for a prospective randomised controlled trial which we expect to initiate in 2025.”
Trauma a strong market for ARX
Based on its estimates, more than 86,000 trauma procedures are performed in the US each year, and the US TAM for those procedures alone is ~US$300m. In many of these cases, Myriad can be used to initiate the healing process.
Ward says from an outcome and economic perspective, the use of Myriad is compelling in trauma cases.
He says with a trauma procedure, the patient has often lost a lot of tissue. This needs to be repaired so that patient can be stabilised, anatomy restored, and healing can be accelerated.
“The feedback from clinicians has been very positive and they like that they can immediately use Myriad in surgery and see rapid tissue formation,” Ward says.
“They are finding Myriad to be cost effective, and this is allowing them to use it in procedures they may not have contemplated before.”
Ward says ARX is putting a lot of focus with its sales team on trauma as a specialty.
“We’re strategically focused on Myriad as a key growth driver, particularly in the trauma segment. Over the next 12 months, we expect to gain momentum, and continue to build clinical evidence supporting the effectiveness of Myriad in trauma procedures.”
At Stockhead, we tell it like it is. While Aroa Biosurgery is a Stockhead advertiser, the company did not sponsor this article.
The post Aroa Biosurgery to target US$300 million trauma market with Myriad products appeared first on Stockhead.