Aussie patent granted to Island Pharma related to flavivirus infections
Chimeric does first patient in Phase 1B clinical trial
Imugene clears milestones in VAXINIA trial
Island Pharma granted Aussie patent
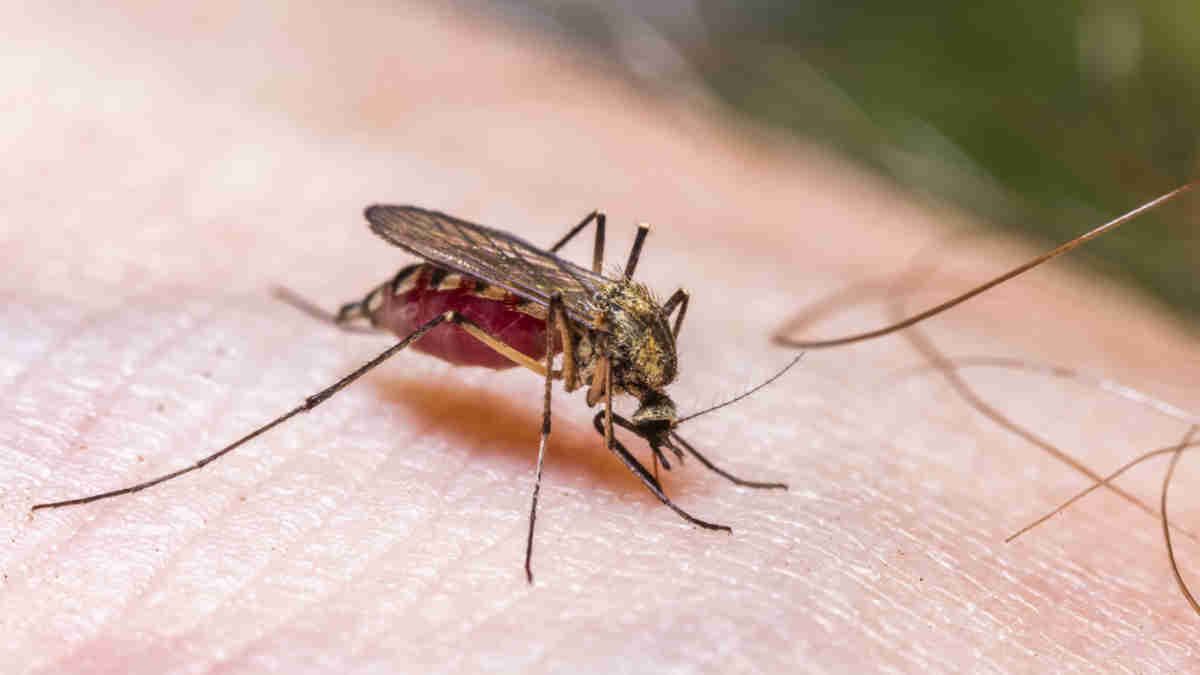
Antiviral drug specialist, Island Pharmaceuticals (ASX:ILA), announced that a key patent relating to its lead drug candidate, ISLA-101, has been granted by IP Australia.
The Australian patent grant entitled “Method of Viral Inhibition” has an expiration date of 16 April 2034. Claims of the patent are directed to the method of treating or preventing flavivirus infections by administering ISLA-101.
Island says the patent underpins its drug repurposing strategy to rapidly develop antiviral therapies, with a key focus being mosquito borne viral diseases, such as dengue fever.
Around 390 million people are infected with dengue fever each year, representing a significant unmet need and opportunity.
The new patent adds to the growing intellectual property thicket that is being established around ISLA-101.
“This Australian patent, together with the equivalent patents granted in other key markets, underpin the investment we are making in bringing forward ISLA-101 as a potential new approach to combatting the world’s growing problem with dengue fever and other mosquito-borne viral diseases,” said Island CEO, Dr David Foster.
This latest Australian patent builds further on Island’s strong IP portfolio that also includes the US, Canada, Brazil, and Singapore.
First patient dose in Chimeric’s Phase 1B clinical trial
Cell therapy biotech, Chimeric Therapeutics (ASX:CHM), says the first patient in the company’s Phase 1B clinical trial in recurrent and/or progressive glioblastoma multiforme (GBM) had received CHM 1101 CAR T cell treatment.
The patient was dosed at the Sarah Cannon Transplant & Cellular Therapy Program at St David’s South Austin Medical Center in Austin, Texas. The patient received CHM 1101 therapy as second line therapy.
The Phase 1B clinical trial for CHM 1101, conducted under a US FDA IND (Investigational New Drug) application, was designed as a two-part clinical trial (Part A, dose confirmation and Part B, dose expansion).
Upon successful completion of the Part B dose expansion cohort, Chimeric will design and initiate a registration trial in collaboration with global regulatory feedback.
“We believe that the full potential of CHM 1101 for patients with recurrent or progressive glioblastoma will only be unlocked through the advancement of our clinical development program, and we look forward to continuing to advance this trial forward,” says Chimeric CEO, Jennifer Chow.
Imugene clears milestones in VAXINIA trial
Meanwhile, Imugene (ASX:IMU) announced that its Phase 1 MAST (metastatic advanced solid tumours) trial evaluating the novel cancer-killing virus CF33- hNIS (VAXINIA) has now cleared certain milestones.
The study has cleared cohort 4 of the intravenous (IV) arm of the monotherapy dose escalation study, as well as IV cohort 2 of the combination study where VAXINIA is administered with blockbuster drug, pembrolizumab (KEYTRUDA).
Cohort 5 of the IV arm for the monotherapy dose escalation is now open, as is IV cohort 3 of the combination study.
Imugene CEO, Leslie Chong, said the company now has an opportunity to expand the trial by enrolling patients in additional cohorts for the monotherapy dose escalation component.
“This will provide us with a far more robust data set to analyse and speak to at the conclusion of the MAST study, and provide us with a stronger platform as we further the clinical development of CF33 and VAXINIA,” she said.
Share prices today:
The post ASX Health Stocks: Island Pharma granted Aussie patent for flavivirus infection drug, ISLA-101 appeared first on Stockhead.






















+ There are no comments
Add yours