Island Pharma establishes dosage level for dengue fever drug
IDT signs deal with global French giant, Sanofi
Telix granted Fast Track by US FDA
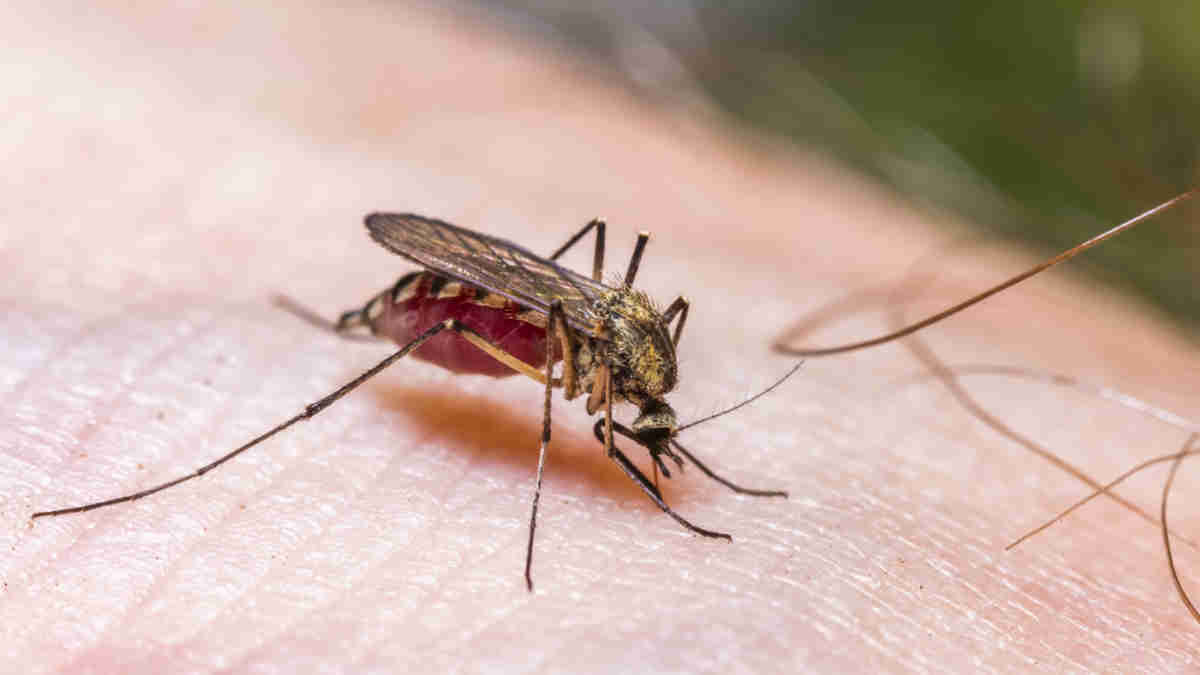
Island Pharma releases data from dengue fever drug
Island Pharma (ASX:ILA) has just reported highly positive pharmacokinetic (PK) data and reconfirmed strong safety/tolerability data for ISLA-101 from its 24-subject Single Ascending Dose clinical study.
ISLA-101 is ILA’s lead asset which is being repurposed for the prevention and treatment of dengue fever and other mosquito (or vector) borne disease.
In its former life, ISLA-101 was the subject of 48 Phase I and II human clinical trials – work which saw it verified as safe in humans by multiple regulators.
Data analysis from the latest study has now confirmed that required levels of ISLA-101 concentration in the blood were observed after only a single dose, achieving the study’s purpose.
This result is critical for determining how the drug acts in the body, and for establishing appropriate dosing regimens for Island’s planned Phase 2a clinical trial.
“This new pharmacokinetic data reinforces our strong confidence in ISLA-101,” said Island’s CEO, Dr David Foster.
“The data has shown us that even following a single dose, we are achieving blood concentrations that have previously been shown to be effective against dengue fever infections.
“This is exactly the outcome we hoped for – it provides a critical datapoint to underpin our dosing regimen as we prepare for the Phase 2 clinical study, which will include dosing for multiple days,” Foster said.
IDT signs $3m+ deal with Sanofi
IDT Australia (ASX:IDT) has entered into a Master Service Agreement with Sanofi, a global French healthcare company, to support the preclinical formulation development and cGMP manufacture of Sanofi’s messenger RNA (mRNA) for its clinical program.
The agreement allows Sanofi to choose services from IDT Australia and allows for follow-on work packages.
The value of the services to be provided under the initial order, which is nearing finalisation, is estimated to be between $3 to 3.5 million (excluding costs relating to storage, shipping and any equipment purchase).
Under the terms, IDT Australia will collaborate with Sanofi to advance the formulation, and manufacture current Good Manufacturing Practice (cGMP) novel mRNA-based vaccines for clinical trials targeting a range of indications.
“This collaboration with Sanofi underscores IDT Australia’s unique expertise and world-class facilities, while reinforcing our position as a trusted partner in the global pharmaceutical industry,” said IDT’s CEO, Paul McDonald.
Telix granted Fast Track by US FDA
Meanwhile in the large end of town, Telix Pharma (ASX:TLX) has been granted a Fast Track Designation by the US FDA for its investigational glioma imaging product, TLX101- CDx.
The Fast Track designation was approved for the characterisation of progressive or recurrent glioma using positron emission tomography (PET).
Concurrently, Telix is in the final stages of preparing its US New Drug Application (NDA) for TLX101-CDx in this initial indication, in both adult and paediatric patients.
The Fast Track designation will enable expedited review and closer consultation with the FDA during that review process.
There is currently no FDA-approved targeted PET agent for brain cancer imaging in the US. Telix’s goal is to make this product commercially available and to significantly increase patient access to this important imaging agent.
Telix has an exclusive research collaboration and data license agreement with the University of California, San Francisco (UCSF), one of the leading academic centres conducting clinical research into the use of PET imaging.
Telix has also selected PharmaLogic as its commercial manufacturing and pharmacy distribution partner, to supply finished unit doses of TLX101-CDx to the US market.
Share prices today:
The post ASX Health Stocks: Island Pharma releases data on dengue fever drug, IDT signs $3m+ deal with Sanofi appeared first on Stockhead.